They contain at least one triple bond between two carbon atoms. Hydrocarbons can be alkanes alkenes or alkynes for alkanes the formula is.

Lesson Explainer Alkynes Nagwa
This is the 2-butyne with the formula C4H6.

. BY reason of their formula alkanes are said to HAVE NO DEGREES of UNSATURATION Where the formula is C_nH_2n or C_nH_2nO_m each 2 hydrogens LESS. The general formula for alkynes is RCCR where R and R are side groups of carbon andor hydrogen atoms bonded together. C n H 2 n 2.
Ethers R-O-R are compounds formed by replacing hydrogen atoms of an alcohol R-OH compound or a phenol C6H5OH by an aryl acyl group functional group after. Alkynes contain four hydrogen atoms less than corresponding alkanes and two hydrogen atoms less than corresponding alkenes and have the general formula C n H 2 n 2. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon.
The general formula of alkynes is eqC_nH_2_n-2 eq where n is an integer number that corresponds to the number of atoms present in a compound. The Below list of methods is used to prepare alkenes. Alkynes react with water in the presence of a catalyst to give.
So you can easily find the general formula of alkynes by inspecting each option by putting the value of n 3. Alkynes are also unsaturated hydrocarbons but these are triple bonded. CnH2n for alkynes the formula is.
What are the uses of alkanes Ans. By partial reduction of alkynes Alkenes can be prepared by reducing alkynes with hydrogen in the presence of a specific catalyst palladised charcoal which is moderately deactivated with the help of quinoline or sulfur compounds. A dialcohol diol b.
How many hydrogens are present on an alkyne with 4 carbons. This group of compounds is a homologous series with the general molecular. An alkynes name ends in.
Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of C_nH_2n-2. They are unsaturated hydrocarbons. The carbon atoms are sp hybridized.
The general formula of alkynes is C n H. On putting the value n3 in. This formula can be.
The general formula of alkynes is C n H 2 n 2 C_nH_2n - 2 C n H 2 n 2. The alkynes comprise a series of carbon and hydrogenbased compounds that contain at least one triple bond. Six hydrogen atoms exist in the moleculeThe general chemical formula of alkynes is CnH2n-2.
The alkynes are unsaturated hydrocarbons that contain one triple bond the general formula of alkynes C n H 2n-2 and the triple bond is known as the acetylenic bond. Alkynes are unsaturated hydrocarbons. We get the chemical.
The general formula of alkynes. The general formula of alkanes is textC_textntextH_2textn 2. General Methods of Preparation of Alkenes.
Alkynes are generally gases and are soluble in. CnH2n2 for alkenes the formula is. This compound can also be prepared from the hydrolysis of calcium carbide a chemical compound with the formula CaC2 also known as calcium acetylide.
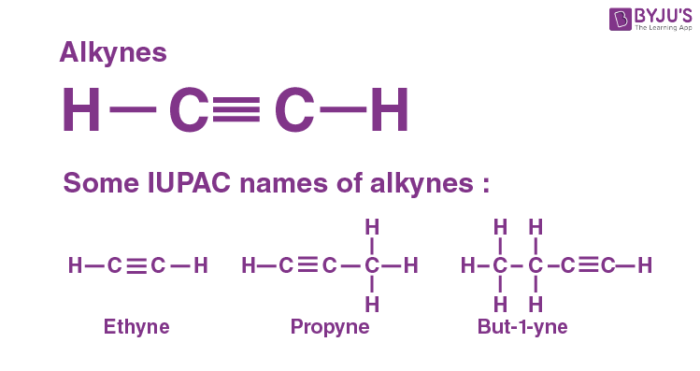
Alkynes Preparation Properties Structure Examples With Videos
Ch105 Chapter 8 Alkenes Alkynes And Aromatic Compounds Chemistry

What Are The Properties Of Alkynes

Class 10 General Formula Of Alkane Alkene Alkyne Tx Academy Youtube
0 Comments